
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o2) are carbon dioxide (co2) and water (h2o).
a mass of 16.74 g for an unknown fuel was combusted in a reaction vessel containing an unknown amount of oxygen. at the end of the reaction, there still remained 16.70 g of the fuel as well as 0.0654 g of water and 0.1198 g of carbon dioxide. the oxygen was completely consumed during the reaction.
how many molecules of oxygen gas were initially present in the reaction vessel?
me, i will be very . the topic is limiting reagents and theoretical yields.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1


Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
As with any combustion reaction, the products of combusting a hydrocarbon fuel (cxhy) with oxygen (o...
Questions



Mathematics, 31.01.2020 01:02

History, 31.01.2020 01:02


Mathematics, 31.01.2020 01:02



English, 31.01.2020 01:02


Mathematics, 31.01.2020 01:02

Mathematics, 31.01.2020 01:02

Biology, 31.01.2020 01:02


Mathematics, 31.01.2020 01:02

Mathematics, 31.01.2020 01:02

Mathematics, 31.01.2020 01:02



Mathematics, 31.01.2020 01:02



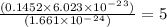 molecules
molecules


