
Chemistry, 18.09.2019 05:30 wowihavefun
A1.0 l sample of an aqueous solution contains 0.10 mol of nacl and 0.10 mol of cacl2. what is the minimum number of moles of agno3 that must be added to the solution in order to precipitate all of the cl- as agcl(s) ? (assume that agcl is insoluble.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
A1.0 l sample of an aqueous solution contains 0.10 mol of nacl and 0.10 mol of cacl2. what is the mi...
Questions

English, 21.08.2021 14:10

Geography, 21.08.2021 14:10

Health, 21.08.2021 14:10

Mathematics, 21.08.2021 14:10

Mathematics, 21.08.2021 14:10


Mathematics, 21.08.2021 14:10

Social Studies, 21.08.2021 14:10

Health, 21.08.2021 14:10


History, 21.08.2021 14:20

English, 21.08.2021 14:20



Mathematics, 21.08.2021 14:20

Computers and Technology, 21.08.2021 14:20


World Languages, 21.08.2021 14:50

Arts, 21.08.2021 14:50

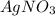 to precipitate all of the
to precipitate all of the 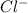 ions.
ions.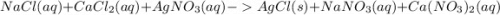
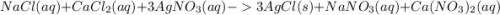
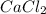 , we need 3 moles of
, we need 3 moles of 

