
Chemistry, 18.09.2019 04:10 maddie7155
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2n2o5(g) → 4no2 (g) + o2 (g) when the rate of formation of no2 is 5.5 ⋅ 10-4 m/s, the rate of decomposition of n2o5 is m/s. at elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2n2o5(g) 4no2 (g) + o2 (g) when the rate of formation of no2 is 5.5 10-4 m/s, the rate of decomposition of n2o5 is m/s. 2.8 ⋅ 10-4 1.4 ⋅ 10-4 5.5 ⋅ 10-4 10.1 ⋅ 10-4 2.2 ⋅ 10-3

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 10:00
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1
You know the right answer?
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2n2o5(g)...
Questions



Computers and Technology, 28.07.2020 21:01







Computers and Technology, 28.07.2020 21:01






Biology, 28.07.2020 21:01




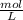 -
-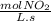 ×
× 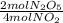 = 2,8 M/s
= 2,8 M/s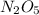 is
is 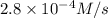
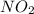 =
= 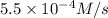
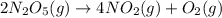
![\text{Rate of decomposition of }N_2O_5=-\frac{1}{2}\times \frac{d[N_2O_5]}{dt}](/tpl/images/0238/6091/c0940.png)
![\text{Rate of formation of }NO_2=+\frac{1}{4}\frac{d[NO_2]}{dt}](/tpl/images/0238/6091/2ef8c.png)
![\text{Rate of formation of }O_2=+\frac{d[O_2]}{dt}](/tpl/images/0238/6091/db5e4.png)
![\text{Rate of reaction}=-\frac{1}{2}\times \frac{d[N_2O_5]}{dt}=+\frac{1}{4}\frac{d[NO_2]}{dt}=+\frac{d[O_2]}{dt}](/tpl/images/0238/6091/52065.png)
![-\frac{1}{2}\times \frac{d[N_2O_5]}{dt}=+\frac{1}{4}\frac{d[NO_2]}{dt}](/tpl/images/0238/6091/5fd92.png)
![-\frac{d[N_2O_5]}{dt}=+\frac{2}{4}\frac{d[NO_2]}{dt}](/tpl/images/0238/6091/ff940.png)
![-\frac{d[N_2O_5]}{dt}=\frac{2}{4}\times (5.5\times 10^{-4}M/s)](/tpl/images/0238/6091/9cde4.png)
![-\frac{d[N_2O_5]}{dt}=2.8\times 10^{-4}M/s](/tpl/images/0238/6091/65aeb.png)


