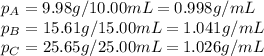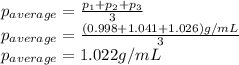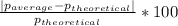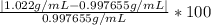Chemistry, 18.09.2019 04:10 christianskyy7074
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0.997655 g/ml. a volume of 10.00 ml of the water had a mass of 9.98 g. a volume of 15.00 ml of the water had a mass of 15.61 g. a volume of 25.00 ml of the water had a mass of 25.65 g. (1) show the calculation of the density of each volume. (2) show the calculation of the average density. (3) show the calculation of the percent error based on the theoretical density of 0.997655 g/ml.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
Density measurements were conducted on a 22.5oc sample of water which had a theoretical density of 0...
Questions

English, 30.12.2020 01:10


Business, 30.12.2020 01:10

Mathematics, 30.12.2020 01:10


Mathematics, 30.12.2020 01:10

Advanced Placement (AP), 30.12.2020 01:10

Business, 30.12.2020 01:10







Geography, 30.12.2020 01:10



Computers and Technology, 30.12.2020 01:10


English, 30.12.2020 01:10