

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 04:00
Silver reacts with oxygen to produce silver oxide. (write balanced chemical equation and identify type of chemical reaction.)
Answers: 1

Chemistry, 23.06.2019 13:00
How long could you survive without electricity? what parts of your life would be affected by loss of electricity? should you prepare for an electricity outage, and if so, how would you prepare? what backup system could your family or community install to generate limited amounts of electricity during an outage? how does this system create an electric force field and generate electric current?
Answers: 2

Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1
You know the right answer?
What is the freezing point of a 1,4-dioxane solution if that same solution boils at 104.6 °c? the n...
Questions













Business, 27.06.2019 04:50

English, 27.06.2019 04:50

Mathematics, 27.06.2019 04:50

English, 27.06.2019 04:50

English, 27.06.2019 04:50

English, 27.06.2019 04:50

Health, 27.06.2019 04:50

 °C
°C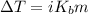
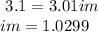
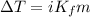
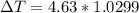
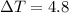 °C
°C

