
Chemistry, 18.09.2019 04:00 Elepeodowke
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.852.85 × 10−4 g mgcl2 in 2.252.25 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:00
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
You know the right answer?
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.852.85 ×...
Questions

Social Studies, 14.01.2021 23:20








Mathematics, 14.01.2021 23:20

Mathematics, 14.01.2021 23:20

Health, 14.01.2021 23:20



Mathematics, 14.01.2021 23:20

Mathematics, 14.01.2021 23:20

Mathematics, 14.01.2021 23:20

Mathematics, 14.01.2021 23:20

Mathematics, 14.01.2021 23:20

Mathematics, 14.01.2021 23:20

Mathematics, 14.01.2021 23:20

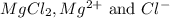 are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively.
are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively.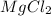 =
= 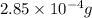
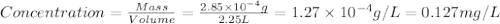
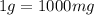
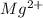 and
and 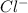 .
.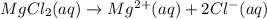
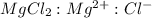 is, 1 : 1 : 2. So,
is, 1 : 1 : 2. So,

