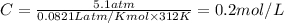The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute temperature and the gas constant r. suppose the osmotic pressure of a certain solution is measured to be 5.1 atm at an absolute temperature of 312 k. write an equation that will let you calculate the molarity of this solution. your equation should contain only symbols. be sure you define each symbol other than r .

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 23.06.2019 05:50
What is the molecular formula of ferrous nitrate and ferric nitrate
Answers: 2

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2
You know the right answer?
The osmotic pressure exerted by a solution is equal to the molarity multiplied by the absolute tempe...
Questions

Mathematics, 06.05.2021 19:40

History, 06.05.2021 19:40

Health, 06.05.2021 19:40

Mathematics, 06.05.2021 19:40



Mathematics, 06.05.2021 19:40

Mathematics, 06.05.2021 19:40


Computers and Technology, 06.05.2021 19:40

Mathematics, 06.05.2021 19:40


Mathematics, 06.05.2021 19:40

Engineering, 06.05.2021 19:40


Mathematics, 06.05.2021 19:40

English, 06.05.2021 19:40


History, 06.05.2021 19:40

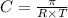

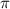 = osmotic pressure = 5.1 atm
= osmotic pressure = 5.1 atm