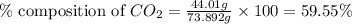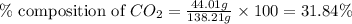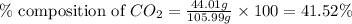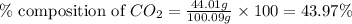A1.1000 gram carbonate sample chosen from li2co3, k2co3, na2co3 and caco3 was reacted with h2so4 and was found to lose 0.3497 gram of co2. (1) show the calculation of the % co2 in the unknown carbonate sample. (2) show the calculation of the % co2 in each of the carbonate compounds and identify the unknown carbonate from the list. atomic weights: c = 12.01, o = 16.00. mws: li2co3 = 73.892, k2co3 = 138.21, na2co3 = 105.99 and caco3 = 100.09

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3
You know the right answer?
A1.1000 gram carbonate sample chosen from li2co3, k2co3, na2co3 and caco3 was reacted with h2so4 and...
Questions

Mathematics, 26.02.2021 08:30

Mathematics, 26.02.2021 08:30

Mathematics, 26.02.2021 08:30



Biology, 26.02.2021 08:30

Mathematics, 26.02.2021 08:30

Mathematics, 26.02.2021 08:30

Health, 26.02.2021 08:30


Chemistry, 26.02.2021 08:30


Mathematics, 26.02.2021 08:30

Mathematics, 26.02.2021 08:30

Mathematics, 26.02.2021 08:30



Physics, 26.02.2021 08:30


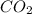 in unknown carbonate sample is 31.79 %
in unknown carbonate sample is 31.79 %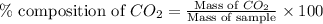 .....(1)
.....(1)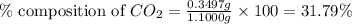
![[1\times 12.01)+(2\times 16.00)]=44.01g](/tpl/images/0237/8770/41a6a.png)