
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is at a total pressure of 150 kpa, and a temperature of 500 k. the mixture has the following composition in mole fraction: 0.25 helium, 0.25 argon, 0.25 krypton. determine: (a) the mass fraction of helium. (b) the average molecular weight of the mixture. (c) the total molar concentration. (d) the mass density.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Amixture of noble gases [helium (mw 4), argon (mw 40), krypton (mw 83.8), and xenon (mw 131.3)] is a...
Questions

Mathematics, 17.05.2021 19:20

English, 17.05.2021 19:20

Mathematics, 17.05.2021 19:20


Mathematics, 17.05.2021 19:20

Mathematics, 17.05.2021 19:20


Mathematics, 17.05.2021 19:20


History, 17.05.2021 19:20



Mathematics, 17.05.2021 19:20


Mathematics, 17.05.2021 19:20



Physics, 17.05.2021 19:20


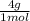 = 1 g of HeliumArgon: 0,25 moles ×
= 1 g of HeliumArgon: 0,25 moles ×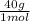 = 10 g of ArgonKrypton: 0,25 moles ×
= 10 g of ArgonKrypton: 0,25 moles ×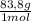 = 20,95 g of krypton Xenon: 0,25 moles ×
= 20,95 g of krypton Xenon: 0,25 moles ×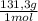 = 32,825 g of Xenon
= 32,825 g of Xenon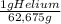 × 100 = 1,6%
× 100 = 1,6%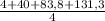 = 64,775 g/mol
= 64,775 g/mol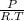 = M
= M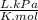
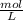 ×
× 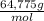 ×
× 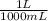 =
=

