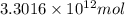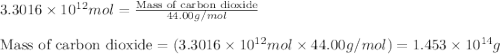Chemistry, 17.09.2019 20:10 samanthasheets8006
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (in grams) of co2 produced by the combustion of 1.80 x 1010 gallons of gasoline (the estimated annual consumption of gasoline in the u. remember, there are 3.785 liters in 1 gallon and assume that isooctane is the only carbon containing component of gasoline. scientific notation can be entered as follows: 1.23 x 1023 = 1.23e23

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 1
You know the right answer?
Assuming gasoline is 89.0% isooctane, with a density of 0.692 g/ml, what is the theoretical yield (i...
Questions

English, 17.09.2021 18:10





English, 17.09.2021 18:10

English, 17.09.2021 18:10

Mathematics, 17.09.2021 18:10


English, 17.09.2021 18:10


English, 17.09.2021 18:20



Mathematics, 17.09.2021 18:20

English, 17.09.2021 18:20

Mathematics, 17.09.2021 18:20



Health, 17.09.2021 18:20

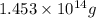
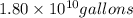
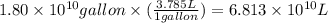
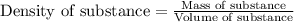
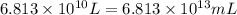 (Conversion factor: 1 L = 1000 mL)
(Conversion factor: 1 L = 1000 mL)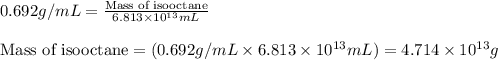
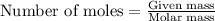 .....(1)
.....(1)
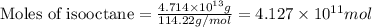
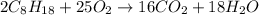
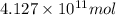 of isooctane will produce =
of isooctane will produce = 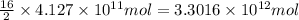 of carbon dioxide
of carbon dioxide