
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is mixed with 11.5 l of o2(g) at 2.66 atm and 298 k.(a) calculate the number of moles of each of the following before the reaction occurs.(i) fe(s)(ii) o2(g)(b) identify the limiting reactant when the mixture is heated to produce fe2o3. support youranswer with calculations.(c) calculate the number of moles of fe2o3 produced when the reaction proceeds tocompletion.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2
You know the right answer?
Iron reacts with oxygen to produce iron(iii) oxide as represented above. a 75.0 g sample of fe(s)is...
Questions



Arts, 05.11.2020 03:40



Mathematics, 05.11.2020 03:40





English, 05.11.2020 03:40




Mathematics, 05.11.2020 03:40

English, 05.11.2020 03:40

Mathematics, 05.11.2020 03:40

Mathematics, 05.11.2020 03:40


Mathematics, 05.11.2020 03:40

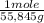 = 1,34 Fe(s) moles
= 1,34 Fe(s) moles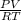 = n
= n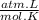
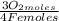 = 1,01 O₂ moles
= 1,01 O₂ moles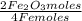 =
= 

