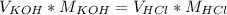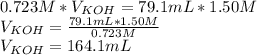Chemistry, 17.09.2019 19:00 chaseking120418
Titration is a type of experiment that can be performed to investigate a neutralization reaction. the equivalence point is when all of the acid and base is fully neutralized. a sample of 0.723 m aqueous potassium hydroxide was titrated against a standard solution of hydrochloric acid. what was the volume of the potassium hydroxide solution if 79.1 ml of 1.50 m hydrochloric acid was needed to reach the equivalence point?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
Use examples from the article to explain one positive and one negative effect that chemistry has had on society
Answers: 2

Chemistry, 21.06.2019 15:30
In this reaction n2o4(g)→2no2(g) what changes in color would you expect as pressure is increased at a constant temperature
Answers: 1

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
Titration is a type of experiment that can be performed to investigate a neutralization reaction. th...
Questions

Biology, 15.07.2019 15:30

Social Studies, 15.07.2019 15:30

History, 15.07.2019 15:30


Business, 15.07.2019 15:30




History, 15.07.2019 15:30





English, 15.07.2019 15:30

Mathematics, 15.07.2019 15:30



Mathematics, 15.07.2019 15:30

Mathematics, 15.07.2019 15:30