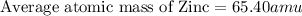2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu; 27.90% of 66zn with an atomic weight of 65.926 amu; 4.10% of 67zn with an atomic weight of 66.927 amu; 18.75% of 68zn with an atomic weight of 67.925 amu; and 0.62% of 70zn with an atomic weight of 69.925 amu. calculate the average atomic weight of zn.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
2.3 zinc has five naturally occurring isotopes: 48.63% of 64 zn with an atomic weight of 63.929 amu...
Questions


Mathematics, 10.05.2021 23:40

Chemistry, 10.05.2021 23:40

Arts, 10.05.2021 23:40

English, 10.05.2021 23:40


Biology, 10.05.2021 23:40

Mathematics, 10.05.2021 23:40

Chemistry, 10.05.2021 23:40






Mathematics, 10.05.2021 23:40





Mathematics, 10.05.2021 23:40

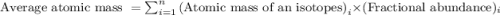 .....(1)
.....(1)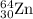 isotope:
isotope: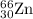 isotope:
isotope: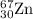 isotope:
isotope: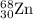 isotope:
isotope: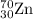 isotope:
isotope:![\text{Average atomic mass of Zinc}=[(63.929\times 0.4863)+(65.926\times 0.2790)+(66.927\times 0.0410)+(67.925\times 0.1875)+(69.925\times 0.0062)]](/tpl/images/0237/0119/27e73.png)