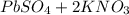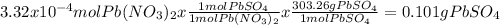Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

You know the right answer?
When aqueous solutions of k₂so₄ and pb(no₃)₂ are combined, pbso₄ precipitates. calculate the mass, i...
Questions



Chemistry, 11.02.2020 20:57

Mathematics, 11.02.2020 20:57

Biology, 11.02.2020 20:57




Mathematics, 11.02.2020 20:57

Mathematics, 11.02.2020 20:57

Biology, 11.02.2020 20:57





History, 11.02.2020 20:58



History, 11.02.2020 20:58

Mathematics, 11.02.2020 20:58

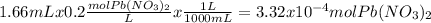
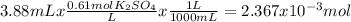
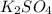
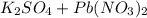 ⇒
⇒