
Chemistry, 17.09.2019 00:10 jewlbug4358
Question 9 suppose 15.3g of sodium bromide is dissolved in 250.ml of a 0.40 m aqueous solution of silver nitrate. calculate the final molarity of bromide anion in the solution. you can assume the volume of the solution doesn't change when the sodium bromide is dissolved in it.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Astudent is asked to identify and element that is pale yellow brittle solid and does not conduct electricity. at which location in this periodic table would the element most likely be found?
Answers: 2


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Question 9 suppose 15.3g of sodium bromide is dissolved in 250.ml of a 0.40 m aqueous solution of si...
Questions












Mathematics, 02.03.2020 23:04








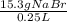 ×
× 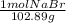 =
= 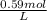 = 0.59 M
= 0.59 M

