
Chemistry, 16.09.2019 22:00 ericahale3971
Suppose 6.54g of potassium bromide is dissolved in 50.ml of a 0.70 m aqueous solution of silver nitrate. calculate the final molarity of bromide anion in the solution. you can assume the volume of the solution doesn't change when the potassium bromide is dissolved in it.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
Suppose 6.54g of potassium bromide is dissolved in 50.ml of a 0.70 m aqueous solution of silver nitr...
Questions


Mathematics, 30.05.2021 02:20

English, 30.05.2021 02:20

Mathematics, 30.05.2021 02:20







Mathematics, 30.05.2021 02:20

Computers and Technology, 30.05.2021 02:20


Mathematics, 30.05.2021 02:20

Mathematics, 30.05.2021 02:20

Physics, 30.05.2021 02:20

Social Studies, 30.05.2021 02:20



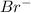 ]=0.4M
]=0.4M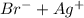 ⇄
⇄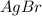 Kps for this reaction is
Kps for this reaction is 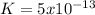 , this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.
, this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.![[Br^{-}]=6.54gKBr.\frac{1molKBr}{119gKBr}.\frac{1molBr^{-} }{1molKBr} .\frac{1}{0.05L} =1.10M](/tpl/images/0234/3240/bfec1.png)
![Qps=[Br^{-}][Ag^{+}]](/tpl/images/0234/3240/78356.png) =
=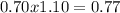
![[Br^{-}]=\frac{(\frac{1.1mol}{L}.0.05L-\frac{0.70mol}{L}.0.05L}{0.05L} =0.4M](/tpl/images/0234/3240/ff056.png)


