
Phosphorus forms several compounds with oxygen, including diphosphorus trioxide and diphosphorus pentoxide. a decomposition of a sample of diphosphorus trioxide forms 1.29 g phosphorus to every 1.00 g oxygen. the decomposition of a sample of diphosphorus pentoxide forms 0.775 g phosphorus to every 1.00 g oxygen. show that these results are consistent with the law of multiple proportions.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is mostly likely why many scientists reject the cold fusion theory
Answers: 1


Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1
You know the right answer?
Phosphorus forms several compounds with oxygen, including diphosphorus trioxide and diphosphorus pen...
Questions

English, 14.07.2020 19:01


Mathematics, 14.07.2020 19:01

Mathematics, 14.07.2020 19:01






Mathematics, 14.07.2020 19:01

Geography, 14.07.2020 19:01


Business, 14.07.2020 19:01

Mathematics, 14.07.2020 19:01



Computers and Technology, 14.07.2020 19:01



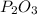 the ratio between P and O is
the ratio between P and O is 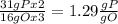 , that is consistent with the experimental result.
, that is consistent with the experimental result.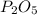 the ratio between P and O is
the ratio between P and O is 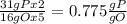 that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.
that is also consistent with experimental results. In conclusion we can say that the results obtained are consistent with the law of multiple proportions.

