
Chemistry, 16.09.2019 17:10 briweaver9993
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2co the reaction of 7.39 kg titanium dioxide with excess c and cl₂ gives 14.24 kg titanium tetrachloride. calculate the theoretical yield of ticl₄ (assuming complete reaction) and its percentage yield.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1


Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
Titanium dioxide, tio₂, reacts with carbon and chlorine to give gaseous ticl₄: tio₂+2c+2ci₂−tici₄+2...
Questions





English, 03.02.2021 01:50


History, 03.02.2021 01:50


Mathematics, 03.02.2021 01:50

Physics, 03.02.2021 01:50

Computers and Technology, 03.02.2021 01:50




Mathematics, 03.02.2021 01:50

Mathematics, 03.02.2021 01:50

Advanced Placement (AP), 03.02.2021 01:50

Mathematics, 03.02.2021 01:50


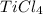 and its percentage yield is 81.0%
and its percentage yield is 81.0%
 =81.0%
=81.0%

