Here's what I find
Explanation:
A. Period or Group?
Na Mg Al Si
These elements are in Period 3.
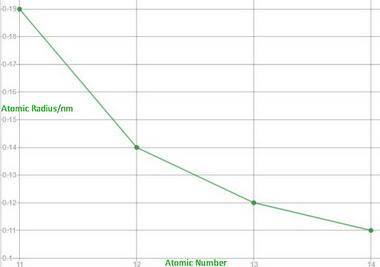
B. Trend in atomic radius

The graph below shows that the atomic radius decreases as the atomic number increases, that is, as you go from left to right across the Period.
C. Explanation
As you go from left to right across a Period, you are adding electrons to the n = 3 shell. The electrons repel each other, so the atoms tend to increase in size.
However, you are also adding protons to the nucleus, so the attraction between the protons and the electrons increases. Each added electron can shield only about 0.3 of a proton's charge, so the nuclear attraction wins out and the atomic radius decreases.