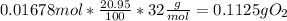Chemistry, 14.09.2019 11:30 loredobrooke9929
At 14,000 ft elevation the air pressure drops to 0.59 atm. assume you take a 1l sample of air at this altitude and compare it to 1 l of air taken at sea level. how much less o2 (in g) is available in 1 l of air at 14,000 ft (assume temperature of 298 k and that relative gas percentages are constant in both locations).

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1
You know the right answer?
At 14,000 ft elevation the air pressure drops to 0.59 atm. assume you take a 1l sample of air at thi...
Questions

English, 02.12.2020 06:10

Mathematics, 02.12.2020 06:10


Mathematics, 02.12.2020 06:20

Spanish, 02.12.2020 06:20


Biology, 02.12.2020 06:20

Biology, 02.12.2020 06:20



Mathematics, 02.12.2020 06:20


Chemistry, 02.12.2020 06:20

English, 02.12.2020 06:20

Mathematics, 02.12.2020 06:20

Arts, 02.12.2020 06:20

Mathematics, 02.12.2020 06:20


History, 02.12.2020 06:20

History, 02.12.2020 06:20