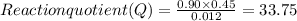Chemistry, 14.09.2019 11:30 tanyadeewill
7. for the system pcls(g) → pc13(g) + cl2(g) kis 26 at 300°c. in a 5.0-l flask, a gaseous mixture consists of all three gases with partial pressure as follows: ppcis = 0.012 atm, pc2=0.45 atm, ppci3 -0.90 atm. a) is the mixture at equilibrium? explain. b) if it is not at equilibrium, which way will the system shift to establish equilibrium?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 17:30
Observation and experimentation have led many scientists to accept a theory about the origin of the universe. this theory is called the big bang theory. scientific evidence collected and observed by scientists around the world suggests that the universe is ever expanding from a hot and dense initial state. what makes this a scientific theory? (2 points)
Answers: 2

You know the right answer?
7. for the system pcls(g) → pc13(g) + cl2(g) kis 26 at 300°c. in a 5.0-l flask, a gaseous mixture co...
Questions

Mathematics, 19.04.2020 22:26

French, 19.04.2020 22:26

Mathematics, 19.04.2020 22:26

Computers and Technology, 19.04.2020 22:26




English, 19.04.2020 22:26

History, 19.04.2020 22:26

Computers and Technology, 19.04.2020 22:26

Social Studies, 19.04.2020 22:26

Mathematics, 19.04.2020 22:26


Health, 19.04.2020 22:26

Biology, 19.04.2020 22:26


Mathematics, 19.04.2020 22:26


Mathematics, 19.04.2020 22:27

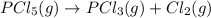
![Reaction\ quotient (Q) = \frac{[p_{PCl_3}]\times [p_{Cl_2}]}{[p_{PCl_5}]}](/tpl/images/0231/3431/4e28d.png)
![[p_{PCl_5}] = 0.012 atm](/tpl/images/0231/3431/6af84.png)
![[p_{PCl_3}]= 0.90 atm](/tpl/images/0231/3431/1d822.png)
![[p_{Cl_2}]= 0.45 atm](/tpl/images/0231/3431/b9835.png)