Chemistry, 14.09.2019 11:10 diamondgray2003
Write a balanced half-reaction for the reduction of permanganate ion (mno) to manganese ion mn? ) in acidic aqueous solution. be sure to add physical state symbols where appropriate. 0-0 cb x 5 2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 12:30
You have 125 g of a certain seasoning and are told that it contains 70.0 g of salt. what is the peroentage of salt by mass in this seasoning?
Answers: 2

Chemistry, 23.06.2019 12:50
Which of these describes the rate of this chemical reaction? h2 + cl2 → 2 hcl a. an increase in the concentration of hcl and h2 with time b. an increase in the concentration of hcl with time c. an increase in h2 and cl2 with time d. a decrease in hcl and cl2 with time
Answers: 1
You know the right answer?
Write a balanced half-reaction for the reduction of permanganate ion (mno) to manganese ion mn? ) in...
Questions

English, 03.02.2021 04:00


Mathematics, 03.02.2021 04:00

Mathematics, 03.02.2021 04:00


Mathematics, 03.02.2021 04:00

Mathematics, 03.02.2021 04:00



Mathematics, 03.02.2021 04:00

English, 03.02.2021 04:00

English, 03.02.2021 04:00

Mathematics, 03.02.2021 04:00

Social Studies, 03.02.2021 04:00



Mathematics, 03.02.2021 04:00

Engineering, 03.02.2021 04:00


Social Studies, 03.02.2021 04:00

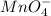 to manganese ion,
to manganese ion, 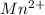 is shown below:
is shown below: