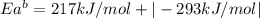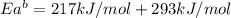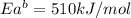Chemistry, 14.09.2019 11:10 joelpimentel
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and the change in enthalpy for the reaction is δh = -293 kj/mol .
what is the activation energy for the reverse reaction?
enter your answer numerically and in terms of kj/mol.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

You know the right answer?
The activation energy for the reaction no2 (g )+ co (g) ⟶ no (g) + co2 (g) is ea = 217 kj/mol and th...
Questions


Mathematics, 29.09.2019 21:10

Mathematics, 29.09.2019 21:10



Biology, 29.09.2019 21:10

Mathematics, 29.09.2019 21:10

Health, 29.09.2019 21:10

Mathematics, 29.09.2019 21:10



History, 29.09.2019 21:10


Mathematics, 29.09.2019 21:10

Physics, 29.09.2019 21:10


History, 29.09.2019 21:10



History, 29.09.2019 21:10

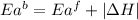
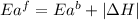
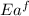 = activation energy for forward reaction
= activation energy for forward reaction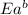 = activation energy for backward reaction
= activation energy for backward reaction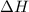 = change in enthalpy of reaction
= change in enthalpy of reaction