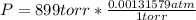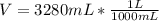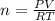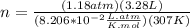part a;
the equation for molarity, m, is
m=n/v
where v is volume and n is the number of moles of solute.
a certain solution has a molarity of m = 2.73 mol/l and has a volume of v = 0.650 l . what is the value of n?
express your answer numerically in moles.
part b;
the equation for photon energy, e, is
e=hcλ
where h = 6.626×10−34 j⋅s (planck's constant) and c = 2.99×108 m/s (the speed of light).
what is the wavelength, λ, of a photon that has an energy of e = 3.98×10−19 j ?
express your answer numerically in meters.
part c;
the ideal gas equation is
pv=nrt
where p is pressure, v is volume, n is the number of moles, r is a constant, and t is temperature.
you are told that a sample of gas has a pressure of p = 899 torr , a volume of v = 3280 ml, and a temperature of t = 307 k . if you use r = 8.206×10−2 l⋅atm/(k⋅mol) , which of the following conversions would be necessary before you could find the number of moles of gas, n, in this sample?
check all that apply.
view available hint(s)
check all that apply.
convert the pressure to atmospheres (atm).
convert the pressure to pascals (pa).
convert the volume to cubic meters (m3).
convert the volume to liters (l).
convert the temperature to degrees celsius (∘c).
convert the temperature to degrees fahrenheit (∘f).

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 09:20
Explain that newton first law,second law and third law of motion?
Answers: 2

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
part a;
the equation for molarity, m, is
m=n/v
where v is volume and n is the num...
the equation for molarity, m, is
m=n/v
where v is volume and n is the num...
Questions

Chemistry, 09.12.2021 06:00

History, 09.12.2021 06:00

Social Studies, 09.12.2021 06:00


Mathematics, 09.12.2021 06:00

English, 09.12.2021 06:00

Biology, 09.12.2021 06:00

Mathematics, 09.12.2021 06:00



Mathematics, 09.12.2021 06:00

Social Studies, 09.12.2021 06:00


Social Studies, 09.12.2021 06:00

Mathematics, 09.12.2021 06:00


Mathematics, 09.12.2021 06:00

English, 09.12.2021 06:00


Mathematics, 09.12.2021 06:00

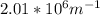
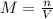
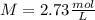 and the volume V = 0.650L, so you need to solve the equation for n:
and the volume V = 0.650L, so you need to solve the equation for n: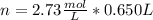
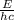
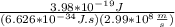
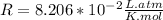 so you need to convert the pressure to atmospheres and convert the volume to liters.
so you need to convert the pressure to atmospheres and convert the volume to liters.