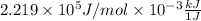Chemistry, 14.09.2019 09:30 victoriadorvilu
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increases to 600. mm hg at 1220.°c. determine the molar heat of vaporization of substance x using the derived form of the clausius-clapeyron equation given below. (include the sign of the value in your answer.) kj/mol

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3
You know the right answer?
The vapor pressure of substance x is 100. mm hg at 1080.°c. the vapor pressure of substance x increa...
Questions

English, 12.10.2019 05:30

Mathematics, 12.10.2019 05:30

Computers and Technology, 12.10.2019 05:30


History, 12.10.2019 05:30





Mathematics, 12.10.2019 05:30

Mathematics, 12.10.2019 05:30

Biology, 12.10.2019 05:30




Mathematics, 12.10.2019 05:30




History, 12.10.2019 05:30

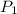 = 100 mm Hg or
= 100 mm Hg or 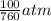 = 0.13157 atm
= 0.13157 atm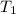 =
= 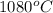 = (1080 + 273) K = 1357 K
= (1080 + 273) K = 1357 K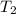 =
= 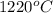 = (1220 + 273) K = 1493 K
= (1220 + 273) K = 1493 K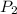 = 600 mm Hg or
= 600 mm Hg or 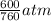 = 0.7895 atm
= 0.7895 atm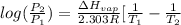
![log(\frac{0.7895}{0.13157}) = \frac{\Delta H_{vap}}{2.303 \times 8.314 J/mol K}[\frac{1}{1357 K} - \frac{1}{1493 K}]](/tpl/images/0231/2242/21278.png)
![log (6) = \frac{\Delta H_{vap}}{19.147}[\frac{(1493 - 1357) K}{1493 K \times 1357 K}]](/tpl/images/0231/2242/46d38.png)
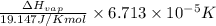
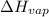 =
= 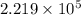 J/mol
J/mol