Chemistry, 14.09.2019 09:30 litwork5885
Hexane is a common laboratory solvent and a component of petrol. its combustion reaction is as follows: 2 c6h14 (9) + 19 02(9) +12 co2(g) + 14 h20(1) arh=-8326 kj mol-1 use the reaction enthalpy value to calculate the heat in (kj) released by combustion of 0.121 mol of hexane. (give your answer as a positive number to at least three significant figures and do not include the units in the answer box)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2
You know the right answer?
Hexane is a common laboratory solvent and a component of petrol. its combustion reaction is as follo...
Questions

English, 25.02.2021 05:40

Business, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40

Chemistry, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40

History, 25.02.2021 05:40


Mathematics, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40


Mathematics, 25.02.2021 05:40



Mathematics, 25.02.2021 05:40



Mathematics, 25.02.2021 05:40

Mathematics, 25.02.2021 05:40

Geography, 25.02.2021 05:40

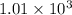
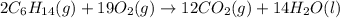
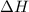 = -8326 kJ/mol
= -8326 kJ/mol