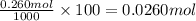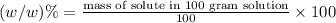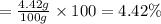Chemistry, 14.09.2019 09:10 toricepeda82
Use the ref an aqueous solution of chromium(ii) acetate has a concentration of 0.260 molal. the percent by mass of chromium(ii) acetate in the solution is submit answer retry entire group 9 more group attempts remaining

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Use the ref an aqueous solution of chromium(ii) acetate has a concentration of 0.260 molal. the perc...
Questions



Health, 07.04.2020 02:52


History, 07.04.2020 02:52


Spanish, 07.04.2020 02:52

Mathematics, 07.04.2020 02:52

Geography, 07.04.2020 02:52



Chemistry, 07.04.2020 02:52



Mathematics, 07.04.2020 02:52



Mathematics, 07.04.2020 02:52