
Chemistry, 14.09.2019 09:10 savannahvargas512
7. aspirin has a pka of 3.4. what is the ratio of a to ha in: (a) the blood (ph 7.4) (b) the stomach (ph 1.4) =

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

You know the right answer?
7. aspirin has a pka of 3.4. what is the ratio of a to ha in: (a) the blood (ph 7.4) (b) the stomac...
Questions

Social Studies, 21.01.2020 01:31



English, 21.01.2020 01:31

Social Studies, 21.01.2020 01:31








English, 21.01.2020 01:31

Computers and Technology, 21.01.2020 01:31






![\frac{[A^-]}{[HA]}=10^{4}](/tpl/images/0231/1723/f3ee2.png)
![\frac{[A^-]}{[HA]}=0.01](/tpl/images/0231/1723/eb40d.png)
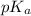 for aspirin = 3.4
for aspirin = 3.4![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0231/1723/e961a.png)
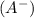 = ?
= ?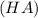 = ?
= ?![pH=pK_a+\log \frac{[A^-]}{[HA]}](/tpl/images/0231/1723/a5883.png)
![7.4=3.4+\log \frac{[A^-]}{[HA]}](/tpl/images/0231/1723/6c4e0.png)
![1.4=3.4+\log \frac{[A^-]}{[HA]}](/tpl/images/0231/1723/643a1.png)


