
Chemistry, 14.09.2019 08:30 Aurionna101
A.) how would you prepare .250 l of a 0.300 m phosphate buffer at ph=3 (h3po4: pka1 = 2.12, pka2 = 7.21, pka = 12.32) using the appropriate weak acid and conjugate salt. b.) how would you prepare the assigned buffer (given in question #1a) if using combining the weak acid and 0.400 m naoh? c.) how would you prepare the assigned buffer (given in question #1a) if using combining the weak acid and 0.400 m hcl?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
A.) how would you prepare .250 l of a 0.300 m phosphate buffer at ph=3 (h3po4: pka1 = 2.12, pka2 =...
Questions

Physics, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00





Mathematics, 17.08.2021 01:00

Chemistry, 17.08.2021 01:00




Mathematics, 17.08.2021 01:00

Computers and Technology, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00



Mathematics, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00

English, 17.08.2021 01:00

Mathematics, 17.08.2021 01:00

![\frac{[H2PO4-]}{[H3PO4]}](/tpl/images/0231/1239/e366e.png)
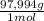 = 0,857 g of H₃PO₄
= 0,857 g of H₃PO₄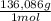 = 9,016 g of KH₂PO₄
= 9,016 g of KH₂PO₄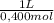 = 0,166 L ≡ 166 mL of 0,400M NaOH
= 0,166 L ≡ 166 mL of 0,400M NaOH

