
Chemistry, 14.09.2019 08:30 webbjalia04
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane and gaseous water in a 0.379 l flask at 1191 k. at equilibrium, the flask contains 0.145 mol of co gas, 0.218 mol of h2 gas, and 0.25 mol of methane. what is the water concentration at equilibrium (kc = 0.30 for this process at 1191 k)?
enter to 4 decimal places.
hint: look at sample problem 17.7 in the 8th ed silberberg book. write a balanced chemical equation. write the kc expression. calculate the equilibrium concentrations of all the species given (moles/liter). put values into kc expression, solve for the unknown.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Omg imgonnafailnfiedkla use complete sentences to explain how the mass of hydrogen is conserved during cellular respiration.
Answers: 1


Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
In a study of the conversion of methane to other fuels, a chemical engineer mixes gaseous methane an...
Questions

Health, 13.11.2021 14:00



Advanced Placement (AP), 13.11.2021 14:00




Business, 13.11.2021 14:00



Mathematics, 13.11.2021 14:00

Social Studies, 13.11.2021 14:00




Mathematics, 13.11.2021 14:00




Mathematics, 13.11.2021 14:00

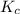
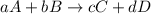
![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0231/1245/b6f47.png)
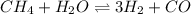
![K_{c}=\frac{[H_2]^3[CO]}{[CH_4][H_2O]}](/tpl/images/0231/1245/e7210.png) ....(1)
....(1)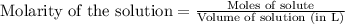
![[CO]=\frac{0.145mol}{0.379L}=0.383mol/L](/tpl/images/0231/1245/315b1.png)
![[H_2]=\frac{0.218mol}{0.379L}=0.575mol/L](/tpl/images/0231/1245/b555b.png)
![[CH_4]=\frac{0.25mol}{0.379L}=0.660mol/L](/tpl/images/0231/1245/35995.png)

![0.30=\frac{(0.575)^3\times 0.383}{0.660\times [H_2O]}](/tpl/images/0231/1245/8e17e.png)
![[H_2O]=\frac{(0.575)^3\times 0.383}{0.660\times 0.30}=0.3677](/tpl/images/0231/1245/f4553.png)


