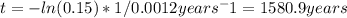Adecomposition reaction has a rate constant of 0.0012 yr^-1
(a) what is the half-life of the r...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
Questions


History, 20.07.2019 13:50

Biology, 20.07.2019 13:50



History, 20.07.2019 13:50

Mathematics, 20.07.2019 13:50




History, 20.07.2019 13:50

Biology, 20.07.2019 13:50

Social Studies, 20.07.2019 13:50

Biology, 20.07.2019 13:50

Computers and Technology, 20.07.2019 13:50





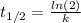
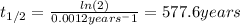
![[A]=[A_{o} ]*e^{-kt}](/tpl/images/0231/1251/24fc7.png)
![t=-ln([A]/[A]_{o}) *1/k](/tpl/images/0231/1251/8a3ec.png)
![0.15*[A_{o}]](/tpl/images/0231/1251/d4ecf.png)