
Chemistry, 14.09.2019 08:30 brianamitzel1013
500 ml of a solution contains 1000 mg of cacl2. molecular weight of cacl2 is 110 g/mol. specific gravity of the solution is 0. cacl2 = ca++ + 2cl-
a) express the concentration of the solution in % w/v
b) express the concentration in ratio strength
c) express the concentration in molarity (m)
d) express the concentration in molality (m)
e) how many equivalents of calcium chloride would be in 1.5 l of the solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 01:00
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
You know the right answer?
500 ml of a solution contains 1000 mg of cacl2. molecular weight of cacl2 is 110 g/mol. specific gra...
Questions

Mathematics, 30.04.2021 17:20



Arts, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20




Mathematics, 30.04.2021 17:20


Mathematics, 30.04.2021 17:20


Mathematics, 30.04.2021 17:20


Physics, 30.04.2021 17:20

Mathematics, 30.04.2021 17:20


History, 30.04.2021 17:20

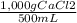 ×100 = 0,2%w/v
×100 = 0,2%w/v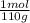 = 9,09×10⁻³ moles of CaCl₂
= 9,09×10⁻³ moles of CaCl₂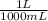 = 0,500 L of solution
= 0,500 L of solution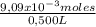 = 0,0182 M
= 0,0182 M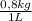 = 0,625 kg of solution
= 0,625 kg of solution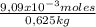 = 0,0145 m
= 0,0145 m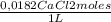 ×
× 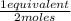 = 0,0137 equivalents
= 0,0137 equivalents

