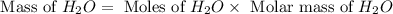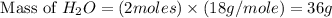Chemistry, 14.09.2019 08:30 anthonyhaywood
For the following reaction, 101 grams of magnesium nitride are allowed to react with 144 grams of water. mg3n2 (5) + 6 h20 (1) — 3 mg(oh)2 (aq) + 2 nh2 (aq) what is the formula for the limiting reagent? what is the maximum amount of magnesium hydroxide that can be formed? grams what amount of the excess reagent remains after the reaction is complete? grams submit answer retry entire group 8 more group attempts remaining

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
For the following reaction, 101 grams of magnesium nitride are allowed to react with 144 grams of wa...
Questions





Mathematics, 07.12.2021 20:00


Mathematics, 07.12.2021 20:00

Mathematics, 07.12.2021 20:00



Mathematics, 07.12.2021 20:00




Mathematics, 07.12.2021 20:00

English, 07.12.2021 20:00


Computers and Technology, 07.12.2021 20:00


SAT, 07.12.2021 20:00

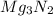 .
.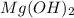 is, 174 grams.
is, 174 grams.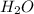 = 144 g
= 144 g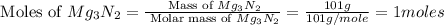
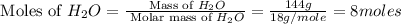
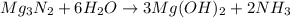
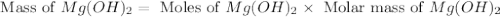
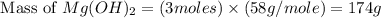
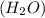 .
.