
Chemistry, 14.09.2019 07:10 Ididntwanttomakethis
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) what is the ph before any naoh is added? b) what is the ph after 5.00 ml of naoh are added? c) after 10 ml of naoh are added? d) what is the ph when 20 ml of naoh have been added? what is this point in the titration called?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1


You know the right answer?
Consider the titration of 100 ml of 0.200 m hcho, with 1.00 m naoh. the pk, of hcho2 is 3.75. a) wha...
Questions


History, 05.01.2020 00:31

Physics, 05.01.2020 00:31




Mathematics, 05.01.2020 00:31

Geography, 05.01.2020 00:31




Computers and Technology, 05.01.2020 00:31

Physics, 05.01.2020 00:31



Mathematics, 05.01.2020 00:31

Mathematics, 05.01.2020 00:31


English, 05.01.2020 00:31

![\frac{[x][x] }{[0,200-x]}](/tpl/images/0231/0228/4f77c.png)
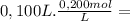 = 2,0x10⁻² mol
= 2,0x10⁻² mol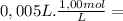 = 5,0x10⁻³ mol
= 5,0x10⁻³ mol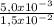
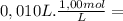 = 1,0x10⁻² mol
= 1,0x10⁻² mol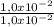
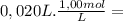 = 2,0x10⁻² mol
= 2,0x10⁻² mol![\frac{[x][x] }{[0,01667-x]}](/tpl/images/0231/0228/2af99.png)



