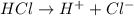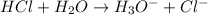Chemistry, 14.09.2019 06:30 theresamarieuehling2
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consider the following acid base reaction hci + h20 → h30+ + cl- a) is this a strong acid? b) clearly label the acid, base, conjugate acid and conjugate base. (5 points)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2


Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1
You know the right answer?
Define the following: bronsted-lowry acid - lewis acid- strong acid - (5 points) problem 6: consid...
Questions




Mathematics, 08.09.2020 04:01



Health, 08.09.2020 04:01



Computers and Technology, 08.09.2020 04:01







Mathematics, 08.09.2020 04:01


History, 08.09.2020 04:01

Mathematics, 08.09.2020 04:01

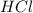 is a strong acid.
is a strong acid. 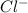 , base =
, base = 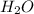 , conjugate acid =
, conjugate acid = 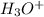
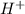 ions.
ions.