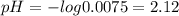Chemistry, 14.09.2019 05:20 selena5713
If a weak acid, ha, is 3% dissociated in a 0.25 m
solution, calculate the ka and the ph of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
If a weak acid, ha, is 3% dissociated in a 0.25 m
solution, calculate the ka and the ph of the...
solution, calculate the ka and the ph of the...
Questions

Mathematics, 15.06.2021 19:10




Chemistry, 15.06.2021 19:10

Mathematics, 15.06.2021 19:10

Chemistry, 15.06.2021 19:10


Biology, 15.06.2021 19:10


Chemistry, 15.06.2021 19:10






Chemistry, 15.06.2021 19:10

History, 15.06.2021 19:10

Mathematics, 15.06.2021 19:10

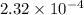
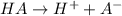
![[H^+]= 0.25\times0.03 = 0.0075 M](/tpl/images/0230/8770/78a73.png)
![[A^{-}]= 0.25 \times 0.03 = 0.0075 M](/tpl/images/0230/8770/ebbeb.png)
![Ka= \frac{[H^+][A^{-}]}{[HA]}](/tpl/images/0230/8770/b86b3.png)
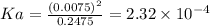
![pH = -log [H^+]](/tpl/images/0230/8770/9b8d2.png)
![H^+[\tex] = 0.0075 M](/tpl/images/0230/8770/51649.png)