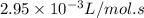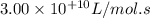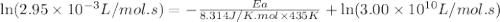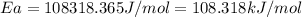Areaction between substances y and z is
by
y2 + z2 > 2yz
the rate constant ob...

Chemistry, 14.09.2019 05:20 johnny2585
Areaction between substances y and z is
by
y2 + z2 > 2yz
the rate constant obeys the arrhenius equation. at 435.
k
the rate constant is k = 2.95 e-03 l/mol-s and a = 3.00 e+10
l/mol-s
what is the activation energy (kj/mol) for
thisreaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3


Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

You know the right answer?
Questions

Mathematics, 09.03.2021 21:00

Physics, 09.03.2021 21:00

History, 09.03.2021 21:00

Mathematics, 09.03.2021 21:00


History, 09.03.2021 21:00

Health, 09.03.2021 21:00


Mathematics, 09.03.2021 21:00



Mathematics, 09.03.2021 21:00

English, 09.03.2021 21:00

Mathematics, 09.03.2021 21:00



Social Studies, 09.03.2021 21:00

History, 09.03.2021 21:00


Arts, 09.03.2021 21:00

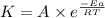
 ............(1)
............(1)