
Chemistry, 25.12.2019 20:31 tommylopez22713
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(g)
all but one of the conditions will cause equilibrium to shift right and produce more of the products. which change will cause the reaction to shift to the left?
a) adding oxygen
b) adding co2
c) increasing the pressure
d) decreasing the temperature

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Anurse practitioner prepares an injection of promethazine, an antihistamine used to treat allergic rhinitis. if the stock bottle is labeled 25 mg/ml and the order is a dose of 11.0 mg , how many milliliters will the nurse draw up in the syringe?
Answers: 3

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1

Chemistry, 23.06.2019 01:00
What type of chemical bond is formed between two atoms of bromine 1. metallic 2. hydrogen 3. ionic 4. covalent
Answers: 1
You know the right answer?
Consider the following reversible, exothermic chemical reaction: 2c2h2(g) + 5o2(g) ↔4co2(g) + 2h2o(...
Questions


History, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31



Business, 16.11.2019 16:31


Mathematics, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31


Mathematics, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31

Social Studies, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31

Mathematics, 16.11.2019 16:31



Mathematics, 16.11.2019 16:31

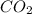
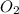 is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of
is increased on reactant side then the equilibrium will shift in the direction where decrease of concentration of 

