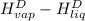Chemistry, 13.09.2019 23:20 SkyeShadow525
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water is 4.19 kj/kg. c, and the heat capacity of water vapor is 1.9 kj/kg-c. h20 at 10 bar boils at 179.9 c. what is the enthalpy of vaporization of h20 at 10 bar? you can neglect the effect of pressure. e 2076 kj/kg e 1924 kj/kg e 2259 kj/kg 2442 kj/kg 2594 kj/kg none of the above

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3


Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
The halpy of vaporization of h2o at 1 atm and 100 c is 2259 kj/kg. the heat capacity of liquid water...
Questions


Mathematics, 26.01.2020 15:31



Mathematics, 26.01.2020 15:31


Mathematics, 26.01.2020 15:31

Chemistry, 26.01.2020 15:31




Mathematics, 26.01.2020 15:31

Mathematics, 26.01.2020 15:31

History, 26.01.2020 15:31

History, 26.01.2020 15:31


Biology, 26.01.2020 15:31


Arts, 26.01.2020 15:31

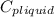 = 4.19
= 4.19 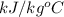
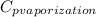 = 1.9
= 1.9 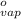 ) at 1 atm and
) at 1 atm and 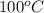 is 2259 kJ/kg
is 2259 kJ/kg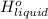 = 0
= 0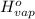 =
= 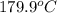 and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and
and effect of pressure is not considered. Hence, enthalpy of liquid water at 10 bar and 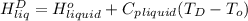
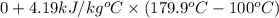
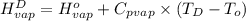
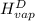 =
= 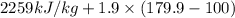
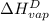 =
=