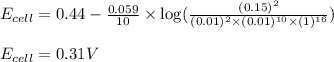Chemistry, 13.09.2019 23:20 michelle230
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius, in which [mno4^1-] = .01m, [br^1-] = .01m, [mn^2+] = .15m, and [h^1+] = 1m. the reaction is 2 mno4^1-(aq) + 10 br^1-(aq) + 16 h^1+(aq) --> 2 mn^2+(aq) + 5 br2(l) + 8 h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

You know the right answer?
Calculate the cell potential for a cell operating with the following reaction at 25 degrees celsius,...
Questions






Arts, 16.10.2020 20:01

History, 16.10.2020 20:01

English, 16.10.2020 20:01

French, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01

English, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01





Mathematics, 16.10.2020 20:01


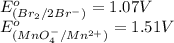
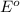 potential will always get reduced and will undergo reduction reaction. Here,
potential will always get reduced and will undergo reduction reaction. Here, 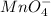 will undergo reduction reaction will get reduced. And, bromine will get oxidized.
will undergo reduction reaction will get reduced. And, bromine will get oxidized.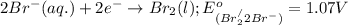 ( × 5)
( × 5)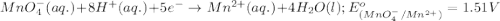 ( × 2)
( × 2)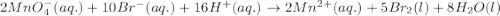
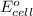 of the reaction, we use the equation:
of the reaction, we use the equation: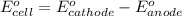
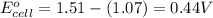
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[Mn^{2+}]^2}{[MnO_4^{-}]^2\times [Br^-]^{10}\times [H^+]^{16}}](/tpl/images/0230/4138/86671.png)
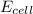 = electrode potential of the cell = ?V
= electrode potential of the cell = ?V![[H^{+}]=1M](/tpl/images/0230/4138/c7b74.png)
![[Mn^{2+}]=0.15M](/tpl/images/0230/4138/f060a.png)
![[MnO_4^{-}]=0.01M](/tpl/images/0230/4138/b97e7.png)
![[Br^{-}]=0.01M](/tpl/images/0230/4138/bb1d7.png)