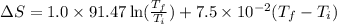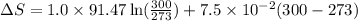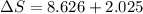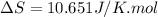in the range 240k to 330k is given

Chemistry, 13.09.2019 23:10 mariaaalopezz
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
bycpm/(jk-1mol-1) = 91.47
+7.5x10-2(t/k). in a particular experiment,
1.0molchcl3 is heated from 273k to 300k. calculate the
changein molar entropy of the sample.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 23.06.2019 08:00
Why is it important for scientists to review and repeat the work of other scientists? 1.a scientific theory must be tested three times before it is proven. 2.the scientific method only applies to repeated experiments. 3.an experiment may have had errors that the scientists didn't recognize. 4.the results of individual scientists may be influenced by bias. 5.an experiment must be performed twice before the data can be analyzed.
Answers: 3

Chemistry, 23.06.2019 08:40
Calculate the number of grams of sodium in 3.00 g of each sodium-containing food additive.
Answers: 3
You know the right answer?
The heat capacity of chloroform (trichloromethane, chcl3)
in the range 240k to 330k is given
in the range 240k to 330k is given
Questions




English, 14.05.2021 21:20

History, 14.05.2021 21:20




Mathematics, 14.05.2021 21:20

History, 14.05.2021 21:20






Mathematics, 14.05.2021 21:20

Biology, 14.05.2021 21:20

Mathematics, 14.05.2021 21:20


Biology, 14.05.2021 21:20

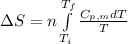
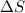 = change in molar entropy
= change in molar entropy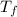 = final temperature = 300 K
= final temperature = 300 K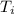 = initial temperature = 273 K
= initial temperature = 273 K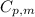 = heat capacity of chloroform =
= heat capacity of chloroform = 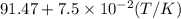
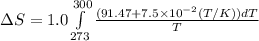
![\Delta S=1.0\times [91.47\ln T+7.5\times 10^{-2}T]^{300}_{273}](/tpl/images/0230/3981/c380e.png)