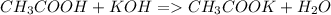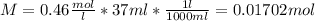25g of vinegar (a solution containing acetic acid) was addedto
a flask containing an indicator...

Chemistry, 13.09.2019 23:10 asialovepink2321
25g of vinegar (a solution containing acetic acid) was addedto
a flask containing an indicator. 37ml of .46m koh solution wasadded
to the system from a burette to reach the equivalence point. what is
the percentage by mass of vinegar that is aceticacid?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1
You know the right answer?
Questions

Mathematics, 22.07.2019 16:30











Business, 22.07.2019 16:30




Social Studies, 22.07.2019 16:30


History, 22.07.2019 16:30


History, 22.07.2019 16:30