
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is burned in pure oxygen, an reaction that proceeds according to the following unbalanced equation.
fex(co)y + o2 --> fe2o3 + co2
if you burn 1.959 g. of fex(co)y and obtain 0.799 g. of fe2o3 and 2.200 g. of co2, what is the empirical formula of fex(co)y?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 13:00
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1

Chemistry, 22.06.2019 21:00
Rays from the sun are not considered matter true or false
Answers: 2
You know the right answer?
To find the formula of a compound composed of iron and carbon monoxide, fex(co)y, the compound is bu...
Questions














Biology, 17.07.2019 22:30


Chemistry, 17.07.2019 22:30

History, 17.07.2019 22:30

Biology, 17.07.2019 22:30


English, 17.07.2019 22:30

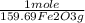 = 5,00×10⁻³ Fe₂O₃ moles
= 5,00×10⁻³ Fe₂O₃ moles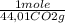 = 5,00×10⁻²CO₂ moles
= 5,00×10⁻²CO₂ moles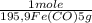 = 1,00×10⁻² Fe(CO)₅ moles
= 1,00×10⁻² Fe(CO)₅ moles

