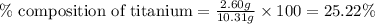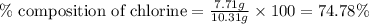Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 10:20
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3


Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1
You know the right answer?
A2.60 g sample of titanium metal chemically combines
withchlorine gas to form 10.31g of a tita...
withchlorine gas to form 10.31g of a tita...
Questions



Mathematics, 03.06.2021 01:00

Social Studies, 03.06.2021 01:00

Mathematics, 03.06.2021 01:00

Business, 03.06.2021 01:00

Mathematics, 03.06.2021 01:00


Mathematics, 03.06.2021 01:00


Chemistry, 03.06.2021 01:00

Mathematics, 03.06.2021 01:00


Chemistry, 03.06.2021 01:00


Mathematics, 03.06.2021 01:00

Mathematics, 03.06.2021 01:00



Chemistry, 03.06.2021 01:00

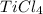
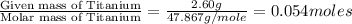
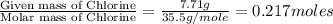
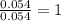
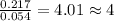
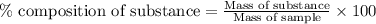 .......(1)
.......(1)