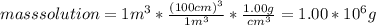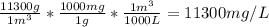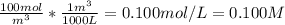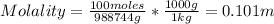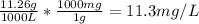Chemistry, 13.09.2019 22:30 maddoxlachowski
The concentration of chlorobenzene (c& hscl) in water is 100 mol/m3. density is 1.00 g/cm3 the solution (a) what is the weight fraction of chlorobenzene? (b) what is the chlorobenzene concentration in ppm? (c) what is the mole fraction of chlorobenzene? (d) what is the molarity of chlorobenzene? (e) what is the molality of chlorobenzene? the concentration of chlorobenzene (c& hscl) in air is 0.100 mol/m3 at 25 °c and 1 atm. the molecular weight of air may be taken to be 28.84 gmol. (a) what is the weight fraction of chlorobenzene? (c) what is the mole fraction of chlorobenzene? (b) what is the chlorobenzene concentration in ppm?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3


Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
The concentration of chlorobenzene (c& hscl) in water is 100 mol/m3. density is 1.00 g/cm3 the s...
Questions


Physics, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00

Engineering, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00





Advanced Placement (AP), 21.09.2021 01:00

Physics, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00

History, 21.09.2021 01:00



Mathematics, 21.09.2021 01:00

Physics, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00

Mathematics, 21.09.2021 01:00