
Chemistry, 13.09.2019 21:20 angel10999
Khoisan salts is the number 1 producer of salts in sa for both local and international markets. 500 kg of kcl is dissolved in sufficient water to make a saturated solution at 370 k. at 370 kthe solubility of kcl is 42 mass %. the solution is cooled to 320 k and the solubility is 31,5 mass % it is assumed that no water is evaporated. 2.1. determine the amount of water is added to the 500 kg of kcl to produce the required saturated solution at 370 k. (3) 2.2. determine the mass of kcl crystals formed after the cooling process to a temperature of 320 k. (use the formula method)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:30
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3

Chemistry, 23.06.2019 07:00
Agas has an empirical formula ch4. 0.16g of the gas occupies a volume of 240cm^3 what is the molecular formula of the me anyone who !
Answers: 1

Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1

Chemistry, 23.06.2019 07:30
In the diagram here that represents the reaction, which reactant, a or b, is the limiting reagent?
Answers: 1
You know the right answer?
Khoisan salts is the number 1 producer of salts in sa for both local and international markets. 500...
Questions

Biology, 29.12.2021 08:30



English, 29.12.2021 08:30

Biology, 29.12.2021 08:30

Biology, 29.12.2021 08:30


SAT, 29.12.2021 08:30

SAT, 29.12.2021 08:30

Mathematics, 29.12.2021 08:30



Social Studies, 29.12.2021 08:30


Engineering, 29.12.2021 08:30


Computers and Technology, 29.12.2021 08:30

Mathematics, 29.12.2021 08:30


History, 29.12.2021 08:40

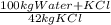 = 1190 kg of water +KCl
= 1190 kg of water +KCl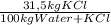 = 375 kg
= 375 kg

