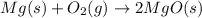When solid magnesium burns, it reacts with oxygen in the air to form a powder called magnesium oxide. a chemist performed this reaction in a lab and found that the mass of the magnesium oxide was greater than the mass of the magnesium. what is the reason behind this increase in mass?
a. the increase in the mass of the magnesium oxide was due to oxygen atoms in the air.
b. the increase in the mass of the magnesium oxide happened because oxygen is heavier than magnesium.
c. the increase in the mass of the magnesium oxide happened because magnesium is heavier than oxygen.
d. the increase in the mass of the magnesium oxide was due to magnesium atoms in the air.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 22.06.2019 21:00
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
When solid magnesium burns, it reacts with oxygen in the air to form a powder called magnesium oxide...
Questions

Medicine, 19.09.2019 04:00


English, 19.09.2019 04:00



English, 19.09.2019 04:00


Physics, 19.09.2019 04:00



History, 19.09.2019 04:00


Mathematics, 19.09.2019 04:00




Business, 19.09.2019 04:00