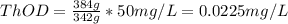Find the theoretical oxygen demand for the
followingsolutions?
a. 200mg/l of acetic aci...

Chemistry, 13.09.2019 17:30 davistakeisha95
Find the theoretical oxygen demand for the
followingsolutions?
a. 200mg/l of acetic acid, ch3cooh.
b. 30mg/l of ethanol, c2h5oh.
c. 50mg/l of sucrose c2h12o6.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

You know the right answer?
Questions

Mathematics, 18.07.2019 05:30



Mathematics, 18.07.2019 05:30


English, 18.07.2019 05:30



Biology, 18.07.2019 05:30

Physics, 18.07.2019 05:30

Mathematics, 18.07.2019 05:30

Mathematics, 18.07.2019 05:30

Mathematics, 18.07.2019 05:30




Mathematics, 18.07.2019 05:30

Mathematics, 18.07.2019 05:30


![[CH3COOH]](/tpl/images/0229/9730/1e5f9.png) = 200 mg/L
= 200 mg/L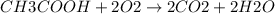
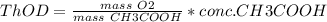
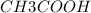 = 60 g
= 60 g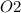 = 2(32) = 64 g
= 2(32) = 64 g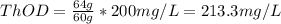
![[C2H5OH]](/tpl/images/0229/9730/4a8a7.png) = 30 mg/L
= 30 mg/L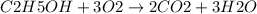
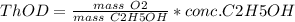
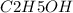 = 46 g
= 46 g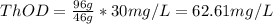
![[C12H22O11]](/tpl/images/0229/9730/ff3eb.png) = 50 mg/L
= 50 mg/L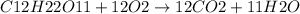
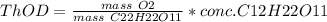
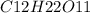 = 342 g
= 342 g