
Chemistry, 12.09.2019 21:30 abbygutierrez401
For a reaction a + b → products, the following data were collected. experiment number initial concentration of a (m) initial concentration of b (m) observed initial rate (m/s) 1 3.40 4.16 1.82 ✕ 10−4 2 4.59 4.16 3.32 ✕ 10−4 3 3.40 5.46 1.82 ✕ 10−4 calculate the rate constant for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

You know the right answer?
For a reaction a + b → products, the following data were collected. experiment number initial concen...
Questions


Geography, 10.09.2021 01:00


Mathematics, 10.09.2021 01:00

Mathematics, 10.09.2021 01:00

English, 10.09.2021 01:00

Mathematics, 10.09.2021 01:00

English, 10.09.2021 01:00


Mathematics, 10.09.2021 01:00

History, 10.09.2021 01:00




Mathematics, 10.09.2021 01:00



Mathematics, 10.09.2021 01:00


Mathematics, 10.09.2021 01:00

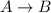
![Rate = k[A]^{x}[B]^{y}](/tpl/images/0229/1912/fc4ee.png)
![\frac{3.32*10^{-4} }{1.82*10^{-4} } =\frac{[4.59]^{x} [4.16]^{y} }{[3.40]^{x} [4.16]^{y} }\\\\x =2](/tpl/images/0229/1912/13d15.png)
![\frac{1.82*10^{-4} }{1.82*10^{-4} } =\frac{[3.40]^{x} [5.46]^{y} }{[3.40]^{x} [4.16]^{y} }\\\\y =0](/tpl/images/0229/1912/8a803.png)
![Rate = k[A]^{2}[B]^{0}](/tpl/images/0229/1912/31aa9.png)
![k = \frac{Rate1}{[A]^{2} } =\frac{1.82*10^{-4}M/s }{[3.40]^{2} } =1.57*10^{-5} s-1](/tpl/images/0229/1912/557a1.png)


