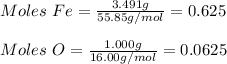Chemistry, 12.09.2019 21:30 dacanul100
Two iron oxide samples are given to you where one is red and the other is black. you perform a chemical analysis and you find that the red sample has a fe/o mass ratio of 2.327 and the black has a fe/o mass ratio of 3.491. you suspect the red sample is simple rust or fe2o3. what is the chemical formula for the black sample?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Two iron oxide samples are given to you where one is red and the other is black. you perform a chemi...
Questions


Biology, 02.08.2019 14:00

Biology, 02.08.2019 14:00







English, 02.08.2019 14:00


Chemistry, 02.08.2019 14:00



Social Studies, 02.08.2019 14:00

Social Studies, 02.08.2019 14:00

English, 02.08.2019 14:00

Chemistry, 02.08.2019 14:00

Mathematics, 02.08.2019 14:00